Time:2024-10-23
Introduction
Today, we will share the research on the stability of Antitumor drug-Ruxolitinib specific impurities. Ruxolitinib is an antitumor drug that has good therapeutic effects on tumor diseases caused by low self immunity, pathogenic bacterial infections, and genetic factors. It can effectively inhibit the growth of tumor cells. In clinical practice, it is mainly used for adult patients with moderate to high-risk primary myelofibrosis (PMF) (also known as chronic idiopathic myelofibrosis), myelofibrosis secondary to polycythemia vera (PPV-MF), or myelofibrosis secondary to primary thrombocytopenia (PET-MF), to treat related diseases, splenomegaly, or disease-related symptoms.
Experimental plan
In this experiment, our center conducted a solution stability study on four specific impurities of Ruxolitinib using the chromatographic conditions used under the "Degradation Products 521-11 and 536-11" and "Related Substances" sections of "Ruxolitinib Phosphate Tablets" (standard number JX20140057). The sample item numbers and structural formulas used are shown in Figure 1 and Figure 2:
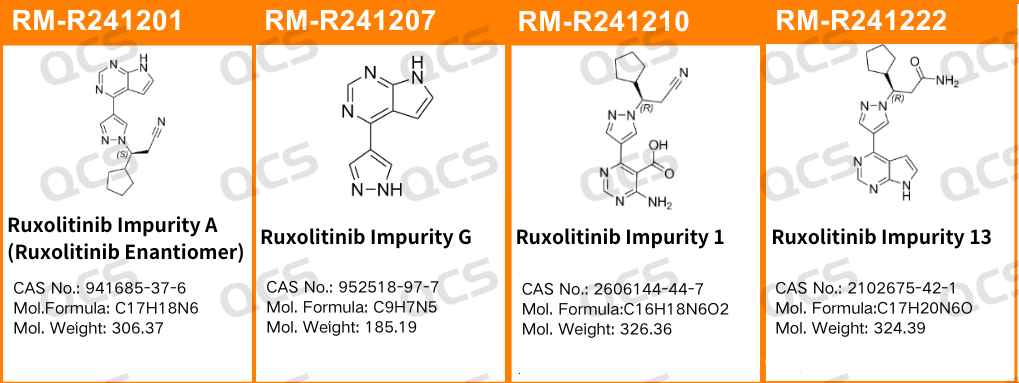
Figure 1: Impurity item numbers and structural formulas used in this study
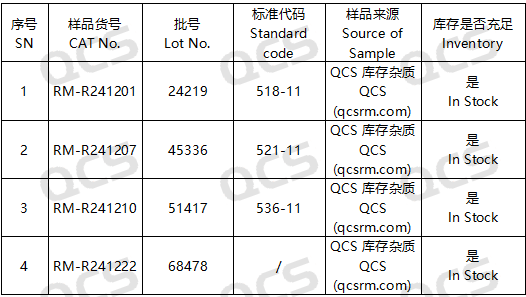
Figure 2: Correspondence between Standard Impurity Code and Impurity Item Number
IIn this experiment, the experimenter took appropriate amounts of RM-R241207 (521-11; Ruxolitinib Impurity G; CAS NO: 952518-97-7) and RM-R241210 (536-11; Ruxolitinib Impurity 1; CAS NO: 2606144-44-7) and placed them in acidic, neutral, and alkaline solutions, respectively. After being placed at room temperature and pressure for 0, 3, 6, 12, and 24 hours, samples were injected for detection according to the chromatographic conditions used in the "Degradation Products 521-11 and 536-11" section of the "Ruxolitinib Phosphate Tablets" (standard number JX20140057). Observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution. Take appropriate amounts of RM-R241201(518-11; Ruxolitinib Impurity A ,Ruxolitinib Enantiomer; CAS NO: 941685-37-6) and RM-R241222 (Ruxolitinib Impurity 13; CAS NO: 2102675-42-1) and place them in acidic, neutral, and alkaline solutions, respectively. After being placed at room temperature and pressure for 0, 3, 6, 12, and 24 hours, samples were injected for detection according to the chromatographic conditions used under the "Related Substances" section of "Ruxolitinib Phosphate Tablets" (standard number JX20140057), Observe the changes in the peak area of the main peak in the chromatogram as the sample solution is left for an extended period of time, and use this as a basis to determine the stability of the sample solution.
Empirical conclusion
RM-R241207(521-11)、RM-R241201(518-11) and RM-R241222
After testing, it was found that the main peak area of samples RM-R241207 (521-11), RM-R241201 (518-11), and RM-R241222 did not change significantly after being placed in acidic, neutral, and alkaline solutions for 24 hours, and the relative standard deviation was less than 2.0%. So it can be considered that the three samples are relatively stable when placed in acidic, neutral, and alkaline solutions for 24 hours. The main peak area data of samples RM-R241207 (521-11), RM-R241201, and RM-R241222 at each detection point under different pH conditions are as follows:
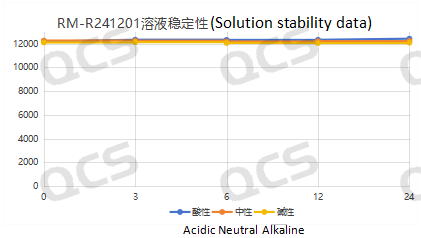
Figure 3: Stability data of sample RM-R241201 (518-11) solution
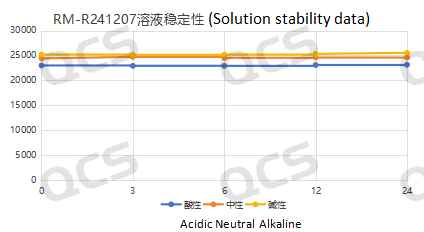
Figure 4: Stability data of sample RM-R241207 (521-11) Solution
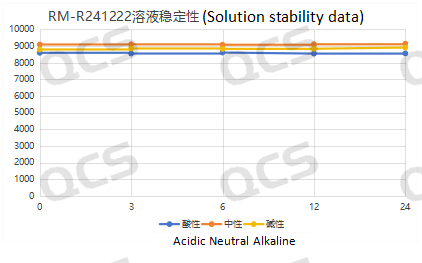
Figure 5: Stability data of sample RM-R241207 (521-11) Solution
After testing, it was found that the main peak area of sample RM-R241210 (536-11) did not change significantly after being placed in acidic and alkaline solutions for 24 hours, and the relative standard deviation was less than 2.0%. So it can be considered that the sample is relatively stable after being placed in acidic and alkaline solutions for 24 hours. The peak area data of the main peak at each detection point of sample RM-R241210 (536-11) under various pH conditions are as follows:
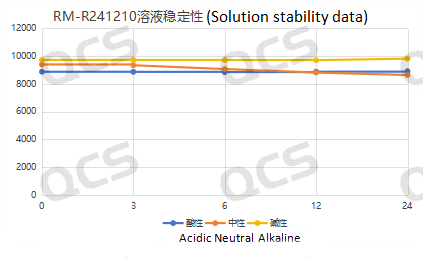
Figure 6: Stability data of sample RM-R241210 (536-11) Solution
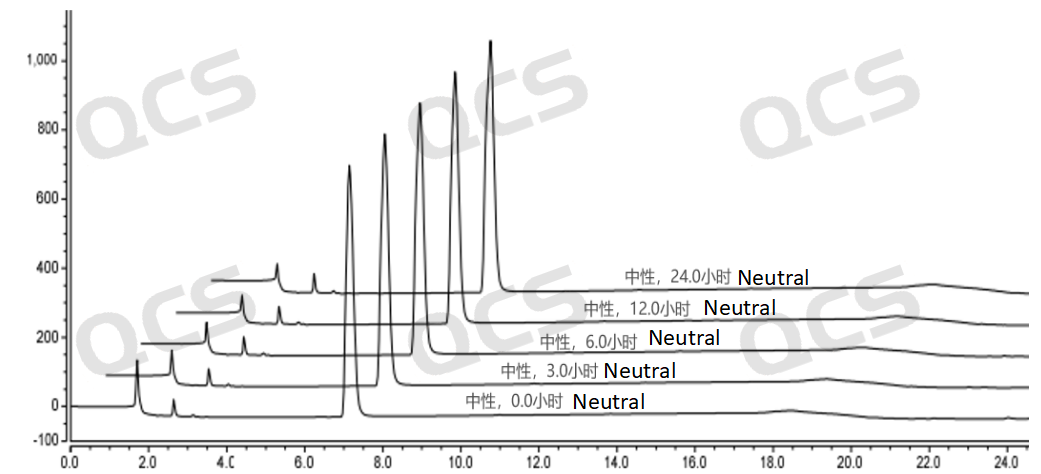
Figure 7: Stability data of neutral solution for sample RM-R241210 (536-11)
Summary
In summary, through this experiment, we found that samples RM-R241207 (521-11), RM-R241201 (518-11), and RM-R241222 have good stability in acidic, alkaline, and neutral solutions. The stability of sample RM-R241210 (536-11) is good in both acidic and alkaline solutions. If customers have a need for the stability of these 4 samples, welcome to consult our company.


CONTACT US
Service hotline: +86 0755-66853366
Marketing Department: +86 136 7004 6396 (WeChat)
Email: sales@chem-strong.com
Office address: Room 1201, Jiaye Plaza, No. 2055, Bixin Road, Longgang District, Shenzhen
*All of our products are for R&D use only, and cannot be used in human or animal clinical use

CONTACT US
Service hotline: +86 0755-66853366
Marketing Department: +86 136 7004 6396 (WeChat)
Email: sales@chem-strong.com
Office address: Room 1201, Jiaye Plaza, No. 2055, Bixin Road, Longgang District, Shenzhen
*All of our products are for R&D use only, and cannot be used in human or animal clinical use