Time:2022-11-03
The company is orchestrating a distinguished conference to celebrate and reflect upon our remarkable achievements over the past decade. It is essential to discuss the significant advancements in generic drug development during this period, particularly focusing on Rivaroxaban raw materials and tablets (marketed under the trade name Barital). At the invitation of our general manger Mr.Qiu, we would like to share some reflections through this public platform, offering insights that serve as a record of our discussions.
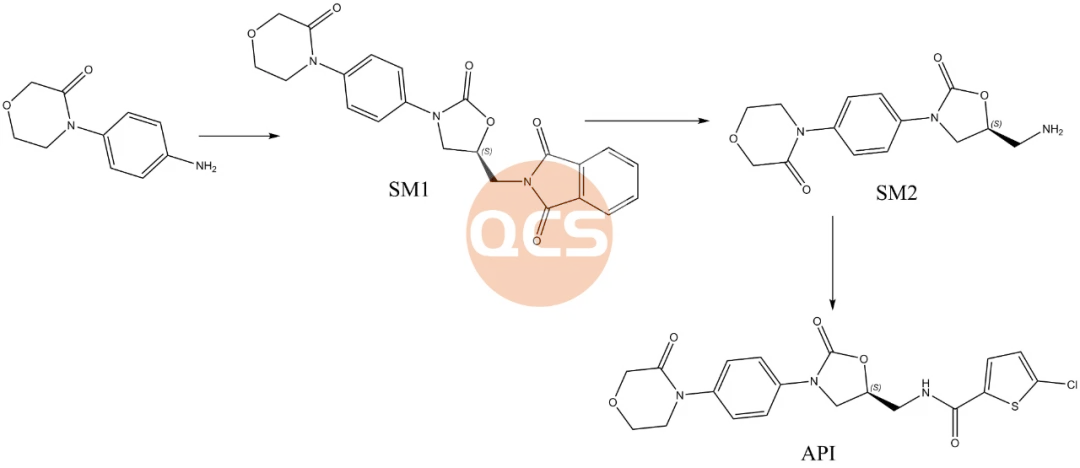
Figure 1: diagram of composite routing
Chapter 1
During the period of 2012-2013, I had the privilege of overseeing the development of our company's Rivaroxaban project. However, in that competitive ‘top thre’ landscape, the initiation of this project lagged nearly a year behind other firms. We successfully obtained quality standards for Rivaroxaban film import registration and established a synthesis route; however, several specific code impurities identified in the standard have emerged as urgent challenges to address. These impurities exhibit distinct relative retention times according to standardized detection methods and can be accurately located and quantified within self-synthesized samples using liquid chromatography. The characterization of these impurities is particularly intriguing—compounds such as acetooxamide, di-oxamido-urea, dechlorination products, oxyphthalimide derivatives, dichlorides, diamines, triamines among others lack precise chemical structure formulas but convey significant information.
Following an extensive brainstorming session, the literature review and speculation regarding potential impurities have been completed alongside synthesis efforts. Through testing of the self-synthesized API sample, four impurities were identified based on their relative retention times and likelihood of introduction, ultimately narrowing down to three remaining impurities: acetooxamide, diamine, and triamine. We will analyze each impurity in detail.
What is the source of the acetyl group in acetooxamide? Regardless of the underlying rationale, based on the successful identification of previous impurities, it can be inferred that intermediate 2 and the acetyl group are linked, and their relative retention times should be analyzed for potential correlation. Indeed, this hypothesis proved to be accurate.
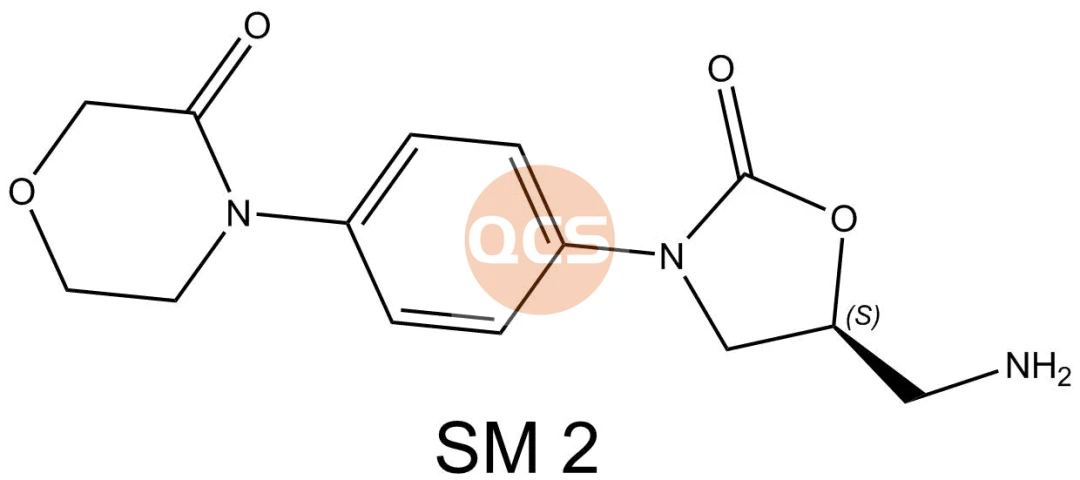
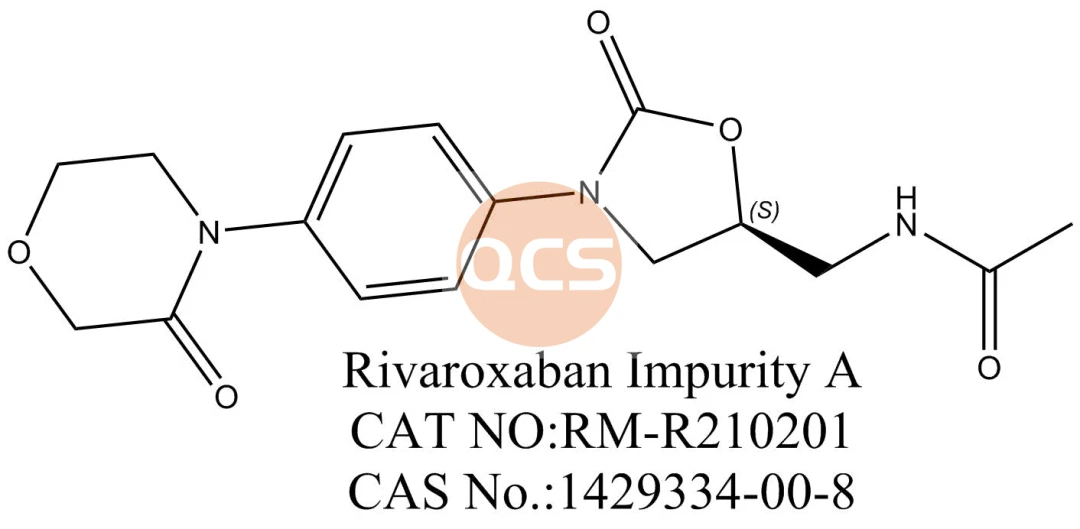
Figure 2: Intermediate 2 and acetyloxamide impurity structure information
Diamines and triamines possess nomenclature reminiscent of dimers or trimers, and they exhibit late peaks, suggesting a potential occurrence. Through self-conducted API testing, it was determined that the sample did not contain these two impurities.
At that time, I conducted all impurity research and process development independently, as the production methods for diamine and triamine were not yet established. Consequently, we initiated a small-scale trial for process optimization and amplification to analyze the impurity spectrum in the final product once the process was fully defined. Simultaneously, some customers were advocating for rivaroxaban's bundled impurities; online forums also engaged in discussions about these two impurities, with reports of a doctor returning from the UK speculating on one of them. Additionally, there was a university professor who insisted on identifying an impurity B as diamine despite being aware that its relative retention time was unknown.

Figure 3: Transmission of diamine structural information within the network
Chapter 2
During the pilot process development, I observed that the solubility of Rivaroxaban API was notably inadequate, with DMSO being the sole solvent available for recrystallization and dissolution filtration, leaving us without an optimal solution. Concurrently, the research and development of rivaroxaban tablets is progressing vigorously. A semi-public document regarding the original formulation of Rivaroxaban raw material COA has raised significant concerns: it lists only three related substances—acetoxamide, diamine, and triamine—yet lacks any structural information! Furthermore, residue testing indicated the presence of acetic acid.
Reflecting on that period, I meticulously sought out information, ensuring that no detail was overlooked. The crude Rivaroxaban API is introduced to acetic acid, which facilitates its complete dissolution at a specific temperature; it is intriguing to consider acetic acid as a solvent for recrystallization. Following several procedures, the test results revealed unexpected impurities in the mother liquor, including acetoxamide, diamine, and triamine. I believe I have gained some insights.
A small quantity of intermediate 2 present in the crude Rivaroxaban API is reacted with acetic acid at elevated temperatures to yield acetooxamide, eliminating the necessity for a shrinkage mixture. Subsequently, through advancements in process optimization, it was established that the crude product from earlier Rivaroxaban synthesis underwent refinement with acetic acid in a controlled environment, resulting in diamine and triamine as process impurities at concentrations of approximately 0.01-0.03%.
What are diamine and triamine? Upon analysis, the concentrations of these two impurities in both the mother liquor and crude products were found to be low, with the mother liquor exhibiting greater complexity. Various experimental processes were conducted to simulate different conditions; ultimately, the levels of these two impurities remained minimal. I have repeatedly performed liquid chromatography to ascertain the molecular weights of these impurities, but all efforts proved futile (details omitted). Time was of the essence, so I selected a batch of approximately 300g of self-synthesized rivaroxaban sample containing ONLY diamine and triamine at concentrations of 0.03% and 0.01%, respectively, for recrystallization. Following multiple rounds of impurity enrichment, two relatively distinct spots appeared on the TLC plate. After scraping the plate several times, only one impurity—likely less than 1 mg—was observed as a thin layer in a 10ml reaction vial. While I was pleased to determine that the relative retention time in liquid chromatography corresponded to diamine, mass spectrometry failed to reveal any significant molecular weight information; additionally, nuclear magnetic resonance spectroscopy only displayed a few prominent peaks while high-field regions appeared chaotic.
Chapter 3
Often, we progress so rapidly that we lose sight of our starting point. How many of you create a comprehensive map detailing all the intermediates in the synthesis process utilizing the same solvent for comparative analysis and identification of potential impurities? I find myself repeatedly sending the mass spectrum in search of reassurance; however, the high-field hydrogen spectrum lacks clarity, prompting me to examine the low-field region instead. How many hydrogens can be identified on each thiophene ring (noting that there are two hydrogens per ring)? There appear to be three! Does this indicate one thiophene fragment or two? Do not hesitate to assert it is two! Excellent. However, there seems to be no available site within the structure for an additional thiophene fragment. What other discrepancies exist? There appears to be an anomaly with morpholine that eludes my observation. Let us consider it as distinct then. This perspective suddenly simplifies matters and opens up new possibilities! Once the structure was elucidated, understanding the mechanism behind impurity formation became apparent: at elevated acidic temperatures, the morpholine ring underwent cleavage while residual traces of thiophenyl chloride and intermediate 2 from prior reactions combined with methylamine resulted in two process-related impurities.


Figure 4: Structural information on Rivaroxaban diamine and triamine impurities.
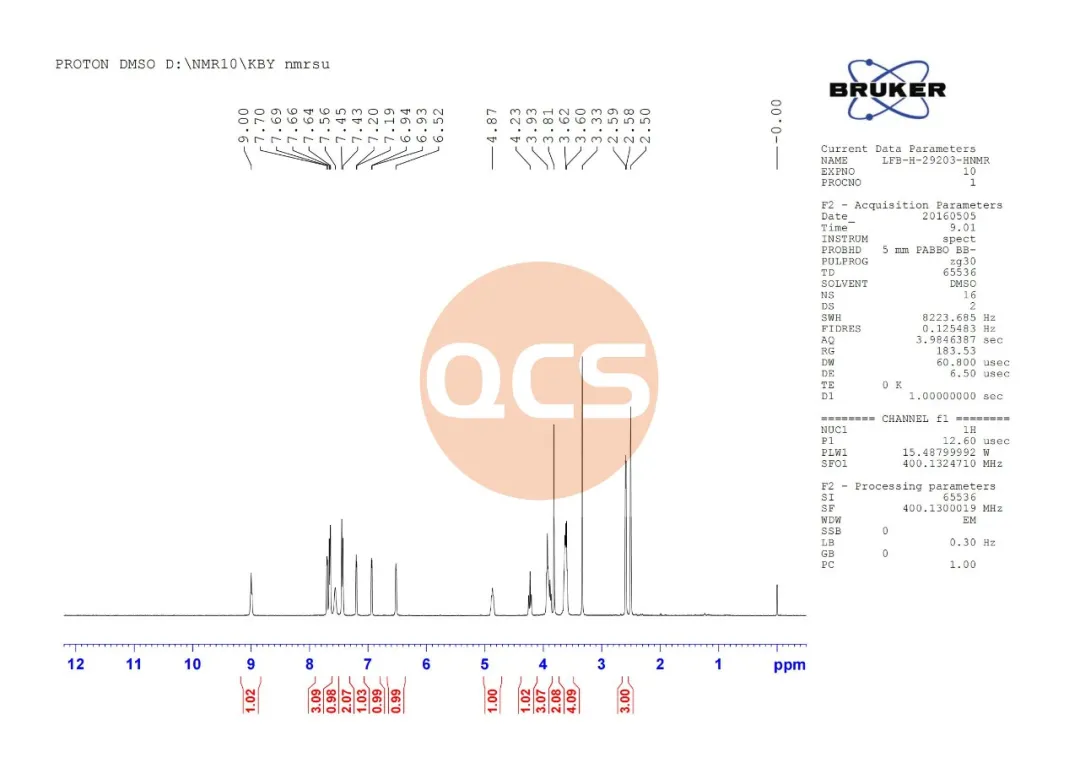
Figure 5: HNMR analysis of Rivaroxaban Diamine
There is no need to elaborate on the background; addressing the diamine to resolve the triamine issue may seem distant. To date, all impurities related to Rivaroxaban's film import registration quality standards have been synthesized directionally. My work with both the process and impurities has taken less than three months, during which I optimized the procedure, thoroughly explained the sources of impurities, and conducted a smooth analysis for quality research. In that context, it can be confidently stated that our research and development efforts were executed with integrity. Naturally, we also consider ourselves among the ‘top three’. However, what more can one achieve?
Reflecting on the past decade, the methodologies and testing conditions for relevant substances in drug development remain relatively underdeveloped. The sources of impurities are often unclear, and there are no commercially available impurity standards. This has shaped a research and development approach centered around ‘process guidance quality’. However, it is encouraging to note that some enterprises continue to pursue research and development aligned with ‘the concept of QBD with Chinese characteristics’.
Today, a decade later, with the advancement of impurity standards by companies such as QCS, there is a wide array of comparative varieties and diverse analytical methods have emerged. Pharmaceutical companies have significantly enhanced their selectivity and information symmetry in drug research and development, thereby accelerating the pace of R&D—a positive development. However, concurrently, it appears that pharmaceutical firms have entered another ‘peculiar cycle’ in drug research and development. Just as several years ago when there was an intense focus on researching impurities, we now observe a vigorous pursuit of gene toxicity impurities; this reflects the so-called competition where strategies and fundamental practices remain largely unchanged.


CONTACT US
Service hotline: +86 0755-66853366
Marketing Department: +86 136 7004 6396 (WeChat)
Email: sales@chem-strong.com
Office address: Room 1201, Jiaye Plaza, No. 2055, Bixin Road, Longgang District, Shenzhen
*All of our products are for R&D use only, and cannot be used in human or animal clinical use

CONTACT US
Service hotline: +86 0755-66853366
Marketing Department: +86 136 7004 6396 (WeChat)
Email: sales@chem-strong.com
Office address: Room 1201, Jiaye Plaza, No. 2055, Bixin Road, Longgang District, Shenzhen
*All of our products are for R&D use only, and cannot be used in human or animal clinical use